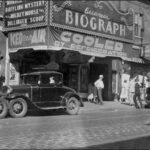Method of Restoration for Ancient Bronzes and other Alloys August 8th, 2020  Cannone nel castello di Haut-Koenigsbourg, photo by Gita Colmar Without any preliminary cleaning the bronze object to be treated is hung as cathode into the 2 per cent. caustic soda solution and a low amperage direct current is applied. The object is suspended with soft copper wires and is completely immersed into the solution. In case the object is very soft and fragile or completely mineralized, fine annealed copper wire is wrapped around the object, one to two turns per inch, and electrical connections are made with several turns of this wire. Where there is danger that object might not hold together upon the removal of the hard supporting shell, we have found it advisable to to pack the whole object in clean white sand, after making proper electrical connections, and then filling the containers with the caustic soda solution.
The anodes are hung on either side of the object. Iron, duriron, and platinum anodes have been used with success. A rectangular glass battery jar of one litre capacity serves well as a container for the treatment of small bronzes. For large objects we have used stoneware tanks and there is no objection to the use of large tanks made of heavy sheet iron welded at the joints.
For a small object of about two to ten square inches of exposed surface, the cell is connected in series with a rheostat and the 110 volts direct current circuit, so as to send from 0.1 to 0.5 amperes through the circuit. A slight gassing at the anode will occur. Sometimes the crust resitance at the point of contact of copper wire is so high that an appreciable current will not pass through the cell at first. Rather than file a clean contact, which is hardly ever to be recommended, it is best to start the cell at night, allow the solution to slowly penetrate the crust and usually be the next morning, the 110 volts potential will be sufficient to have broken through the submerged crust without injury to the surface. Often wetting the copper wire contact with electrolyte will break down the dry resistance of the crust without injury to the surface. Often wetting the copper wire contact with electrolyte will break down the dry resistance of the crust at that point. The object being treated is always made cathode.
The action of the electrolysis is to evolve hydrogen at the cathode and to so reduce the crust to finely divided or spongy copper. This is effectively accomplished in the caustic soda solution. At times reguline copper metal will be deposited in place. Very low current densities are preferred.
The reduction of a thin crust 1/16 to 1/8 inch thick usually requires three or four days. In the case of clayey crusts it is a good plan to change the electrolyte once every twenty-four hours. The use of too strong an electrolyte, or too high a current density will cause excessive gassing at the cathode (the object being treated) and may give rise to warping and falling apart of the object unless it is underlaid by a strong metal core.
Complete reduction is indicated by a free evolution of gas at the cathode with comparatively low current values. The object is then removed from the solution and carefully washed by soaking or steeping in several changes of warm water. This will remove all but traces of caustic soda.
We now arrive at a stage in the process where we must use judgement enough to modify subsequent treatment to fit the general appearance and physical strength of the specimen. If the object treated originally had a hard metal surface under a thin sandy layer of crust, we my remove the reduced copper film or layer by a gentle brushing with a stiff bristle brush or soft brass-wire brush. If, however, examination preliminary to electrolytic treatment indicates that no true metallic core is present, we do not dare subject the treated surfaces to pressure or friction of any kine. In this case a weak acid dip may be necessary and is used as follows:
After thorough washing to remove all but traces of caustic soda, carefully dip the reduce object in to dilute nitric acid (one part acid to four parts of water). There will result a rapid action and evolution of gases, as the reduced outer layer of copper dissolves away, we bring into view the hard, brownish-colored copper oxide surface which preserves the detail of design. This oxide layer does not dissolve readily in the weak acid used, but takes on a greyish tint (due probably to intermixed tin compounds). Inspection with a magnifying glass will show when the treatment has proceeded far enough. It is an exciting moment when we see the the copper dissolve, showing a smooth surface, or design, beneath. The oxide surfaces now exposed are almost as smooth to the touch as metallic surfaces and they will take on a greenish tint or patina when dipped into dilute ammonium acetate and dried in an oven at 40 to 60 Centigrade (104 to 140 Fahrenheit).
Sometimes, especially if the original surface shows cracks in the patina or conspicuously high lumps or “boils” of crust, we would find the underlying oxide layer pitted and roughened in spots if we had used the weak acid dip. Such regions have been highly corroded by localized action and the treatment in our electrolytic bath causes brown copper to fill the pits. Therefore in these cases the weak acid dip is omitted.
Source: The Restoration of Ancient Bronzes and Other Alloys by Colin G. Fink, Ph. D. Professor of Electro-Chemistry at Columbia University and Charles H. Eldridge, B.S. First Report, 1925, The Metropolitan Museum of Art
Home
Top of Pg.
|
Fed Chariman William McChesney Martin – 1952-1970 [Editor note: This response in my mind is quite hilarious…and to the point…who the heck would want to give up 6% interest year after year after year after year? ] You HAVE ASKED that I appear before you today in connection with your consideration [...] Read more →
FRIED SQUIRREL & BISCUIT GRAVY 3-4 Young Squirrels, dressed and cleaned 1 tsp. Morton Salt or to taste 1 tsp. McCormick Black Pepper or to taste 1 Cup Martha White All Purpose Flour 1 Cup Hog Lard – Preferably fresh from hog killing, or barbecue table Cut up three to [...] Read more →
The Effect of Magnetic Fields on Wound Healing Experimental Study and Review of the Literature Steven L. Henry, MD, Matthew J. Concannon, MD, and Gloria J. Yee, MD Division of Plastic Surgery, University of Missouri Hospital & Clinics, Columbia, MO Published July 25, 2008 Objective: Magnets [...] Read more →
Looking to spice up your dinner? Let’s hop along and cook some roo. Home Top of Pg. Read more →
Twinings London – photo by Elisa.rolle Is the tea in your cup genuine? The fact is, had one been living in the early 19th Century, one might occasionally encounter a counterfeit cup of tea. Food adulterations to include added poisonings and suspect substitutions were a common problem in Europe at [...] Read more →
New York Stock Exchange Floor September 26,1963 The Specialist as a member of a stock exchange has two functions.’ He must execute orders which other members of an exchange may leave with him when the current market price is away from the price of the orders. By executing these orders on behalf [...] Read more →
Home Top of Pg. Read more →
Home Top of Pg. Read more →
Sucker The components of any given market place include both physical structures set up to accommodate trading, and participants to include buyers, sellers, brokers, agents, barkers, pushers, auctioneers, agencies, and propaganda outlets, and banking or transaction exchange facilities. Markets are generally set up by sellers as it is in their [...] Read more →
Mocking Bird Food. Hemp seed……….2 pounds Rape seed………. .1 pound Crackers………….1 pound Rice…………….1/4 pound Corn meal………1/4 pound Lard oil…………1/4 pound Home Top of Pg. Read more →
THE SNIPE, from the Shooter’s Guide by B. Thomas – 1811 AFTER having given a particular description of the woodcock, it will only. be necessary to observe, that the plumage and shape of the snipe is much the same ; and indeed its habits and manners sets bear a great [...] Read more →
Armorial tablet of the Stewarts – Falkland Palace Fife, Scotland. The Stewart Kings – King James I & VI to Charles II Six video playlist on the Kings of England: Home Top of Pg. Read more →
Click here to visit Ovation Guitars Ovation Patent Drawing 1975 Click here to read a copy of the 1975 Patent for the Ovation Guitar Home Top of Pg. Read more →
Home Top of Pg. Read more →
PEACH BRANDY 2 gallons + 3 quarts boiled water 3 qts. peaches, extremely ripe 3 lemons, cut into sections 2 sm. pkgs. yeast 10 lbs. sugar 4 lbs. dark raisins Place peaches, lemons and sugar in crock. Dissolve yeast in water (must NOT be to hot). Stir thoroughly. Stir daily for 7 days. Keep [...] Read more →
Reprint from The Pitfalls of Speculation by Thomas Gibson 1906 Ed. THE PUBLIC ATTITUDE TOWARD SPECULATION THE public attitude toward speculation is generally hostile. Even those who venture frequently are prone to speak discouragingly of speculative possibilities, and to point warningly to the fact that an overwhelming majority [...] Read more →
Aw, the good old days, meet in the coffee shop with a few friends, click open the Zippo, inhale a glorious nosegay of lighter fluid, fresh roasted coffee and a Marlboro cigarette…. A Meta-analysis of Coffee Drinking, Cigarette Smoking, and the Risk of Parkinson’s Disease We conducted a [...] Read more →
Click here to learn more about The Tallis Scholars Home Top of Pg. Read more →
Over the years I have observed a decline in manners amongst young men as a general principle and though there is not one particular thing that may be asserted as the causal reason for this, one might speculate… Self-awareness and being aware of one’s surroundings in social interactions [...] Read more →
Roger Scruton – Why Beauty Matters (2009) from Mirza Akdeniz on Vimeo. Click here for another site on which to view this video. Sadly, Sir Roger Scruton passed away a few days ago—January 12th, 2020. Heaven has gained a great philosopher. Home Top of [...] Read more →
Home Top of Pg. Read more →
Cannone nel castello di Haut-Koenigsbourg, photo by Gita Colmar Without any preliminary cleaning the bronze object to be treated is hung as cathode into the 2 per cent. caustic soda solution and a low amperage direct current is applied. The object is suspended with soft copper wires and is completely immersed into [...] Read more →
To Clean Watch Chains. Gold or silver watch chains can be cleaned with a very excellent result, no matter whether they may be matt or polished, by laying them for a few seconds in pure aqua ammonia; they are then rinsed in alcohol, and finally. shaken in clean sawdust, free from sand. [...] Read more →
Take the large blue figs when pretty ripe, and steep them in white wine, having made some slits in them, that they may swell and gather in the substance of the wine. Then slice some other figs and let them simmer over a fire in water until they are reduced [...] Read more →
Click here to view a copy of Arban’s Complete Conservatory Method for Cornet Click on the blue button to download a free copy of Arban’s Complete Conservatory Method for Cornet Arban's - 11.8MB For trumpet players wishing to practice daily using an iPad, simply click [...] Read more →
The arsenicals (compounds which contain the heavy metal element arsenic, As) have a long history of use in man – with both benevolent and malevolent intent. The name ‘arsenic’ is derived from the Greek word ‘arsenikon’ which means ‘potent'”. As early as 2000 BC, arsenic trioxide, obtained from smelting copper, was used [...] Read more →
July 9, 1898. Forest and Stream Pg. 25 Some Notes on American Ship-Worms. [Read before the American Fishes Congress at Tampa.] While we wish to preserve and protect most of the products of our waters, these creatures we would gladly obliterate from the realm of living things. For [...] Read more →
A terrestial globe on which the tracts and discoveries are laid down from the accurate observations made by Capts Cook, Furneux, Phipps, published 1782 / globe by John Newton ; cartography by William Palmer, held by the State Library of New South Wales The British Library, using sophisticated filming equipment and software, [...] Read more →
The Chicago portion of the The Great Train Story at the Museum of Science and Industry in Chicago. by Interiority Home Top of Pg. Read more →
Dominion, Royal St. Lawrence Yacht Club,Winner of Seawanhaka Cup, 1898. The Tail Wags the Dog. The following is a characteristic sample of those broad and liberal views on yachting which are the pride of the Boston Herald. Speaking of the coming races for the Seawanhaka international challenge cup, it says: [...] Read more →
Home Top of Pg. Read more →
As reported in the The Colac Herald on Friday July 17, 1903 Pg. 8 under Art Appreciation as a reprint from the Westminster Gazette ART APPRECIATION IN THE COMMONS. The appreciation of art as well as of history which is entertained by the average member of the [...] Read more →
IT requires a far search to gather up examples of furniture really representative in this kind, and thus to gain a point of view for a prospect into the more ideal where furniture no longer is bought to look expensively useless in a boudoir, but serves everyday and commonplace need, such as [...] Read more →
Home Top of Pg. Read more →
The following highly collectible Franklin Library Signed Editions were published between 1977 and 1982. They are all fully leather bound with beautiful covers and contain gorgeous and rich silk moire endpapers. Signatures are protected by unattached tissue inserts. The values listed are average prices that were sought by [...] Read more →
Home Top of Pg. Read more →
Home Top of Pg. Read more →
? This video by AT Restoration is the best hands on video I have run across on the basics of classic upholstery. Watch a master at work. Simply amazing. Tools: Round needles: https://amzn.to/2S9IhrP Double pointed hand needle: https://amzn.to/3bDmWPp Hand tools: https://amzn.to/2Rytirc Staple gun (for beginner): https://amzn.to/2JZs3x1 Compressor [...] Read more →
Gilbert Stewart – Sir Joshua Reynolds SIR JOSHUA REYNOLDS‘ WORKING COLOURS, WITH THE ORDER IN WHICH THEY WERE ARRANGED ON HIS PALLETTE. “For painting the flesh, black, blue black, white, lake, carmine, orpiment, yellow ochre, ultramarine, and varnish. “To lay the [...] Read more →
Wojna Kalmarska – 1611 The Kalmar War From The Historian’s History of the World (In 25 Volumes) by Henry Smith William L.L.D. – Vol. XVI.(Scandinavia) Pg. 308-310 The northern part of the Scandinavian peninsula, as already noticed, had been peopled from the remotest times by nomadic tribes called Finns or Cwenas by [...] Read more →
Eadweard Muybridge was a fascinating character. Click here to learn how Eadweard committed “Justifiable Homicide” after shooting his wife’s lover in 1874. Home Top of Pg. Read more →
Home Top of Pg. Read more →
Resolution adapted at the New Orleans Convention of the American Institute of Banking, October 9, 1919: “Ours is an educational association organized for the benefit of the banking fraternity of the country and within our membership may be found on an equal basis both employees and employers; [...] Read more →
There is nothing more delightful than a great poetry reading to warm ones heart on a cold winter night fireside. Today is one of the coldest Valentine’s days on record, thus, nothing could be better than listening to the resonant voice of Robin Shuckbrugh, The Cotswold [...] Read more →
From the classic British Movie, The Shooting Party, a 1985 British drama film directed by Alan Bridges based on Isabel Colegate’s 9th novel of the same name published in 1980 we find a scene set in the billiards parlor whereupon the host of the weekend shooting party Sir Randolph Nettleby walks in [...] Read more →
*note – Billesdon and Billesden have both been used to name the hunt. BILLESDEN COPLOW POEM [From “Reminiscences of the late Thomas Assheton Smith, Esq”] The run celebrated in the following verses took place on the 24th of February, 1800, when Mr. Meynell hunted Leicestershire, and has since been [...] Read more →
Como dome facade – Pliny the Elder – Photo by Wolfgang Sauber Work in Progress… THE VARNISHES. Every substance may be considered as a varnish, which, when applied to the surface of a solid body, gives it a permanent lustre. Drying oil, thickened by exposure to the sun’s heat or [...] Read more →
Nov. 12, 1898 Forest and Stream Pg. 396 The Veterans to the Front. Ironton. O., Oct. 28.—Editor Forest and Stream: I mail you a target made here today by Messrs. E. Lawton, G. Rogers and R. S. Dupuy. Mr. Dupuy is seventy-four years old, Mr. Lawton seventy-two. Mr. Rogers [...] Read more →
Crewe Hall Dining Room THE transient tenure that most of us have in our dwellings, and the absorbing nature of the struggle that most of us have to make to win the necessary provisions of life, prevent our encouraging the manufacture of well-wrought furniture. We mean to outgrow [...] Read more →
VHF Marifoon Sailor RT144, by S.J. de Waard RADIO INFORMATION FOR BOATERS Effective 01 August, 2013, the U. S. Coast Guard terminated its radio guard of the international voice distress, safety and calling frequency 2182 kHz and the international digital selective calling (DSC) distress and safety frequency 2187.5 kHz. Additionally, [...] Read more →
ORIGIN OF THE APOTHECARY. The origin of the apothecary in England dates much further back than one would suppose from what your correspondent, “A Barrister-at-Law,” says about it. It is true he speaks only of apothecaries as a distinct branch of the medical profession, but long before Henry VIII’s time [...] Read more →
It is unnecessary to point out that low-grade fruit may often be used to advantage in the preparation of vinegar. This has always been true in the case of apples and may be true with other fruit, especially grapes. The use of grapes for wine making is an outlet which [...] Read more →
St.Helen’s on the Thames, photo by Momit From a Dictionary of the Thames from Oxford to the Nore. 1880 by Charles Dickens Abingdon, Berkshire, on the right bank, from London 103 3/4miles, from Oxford 7 3/4 miles. A station on the Great Western Railway, from Paddington 60 miles. The time occupied [...] Read more →
Laurens’ portrait as painted during his time spent imprisoned in the Tower of London, where he was kept for over a year after being captured at sea while serving as the United States minister to the Netherlands during the Revolutionary War. The first Christian white man to be cremated in America was [...] Read more →
Home Top of Pg. Read more →
Bess of Harwick Four times the nuptial bed she warm’d, And every time so well perform’d, That when death spoil’d each husband’s billing, He left the widow every shilling. Fond was the dame, but not dejected; Five stately mansions she erected With more than royal pomp, to vary The prison of her captive When [...] Read more →
Home Top of Pg. Read more →
What follows is a chapter from Nathaniel Bagshaw Ward’s 1852 treatise on terrarium gardening. ON THE NATURAL CONDITIONS OF PLANTS. To enter into any lengthened detail on the all-important subject of the Natural Conditions of Plants would occupy far too much space; yet to pass it by without special notice, [...] Read more →
THE FOWLING PIECE, from the Shooter’s Guide by B. Thomas – 1811. I AM perfectly aware that a large volume might be written on this subject; but, as my intention is to give only such information and instruction as is necessary for the sportsman, I shall forbear introducing any extraneous [...] Read more →
Home Top of Pg. Read more →
July 2, 1898 Forest and Stream, Fresh-Water Angling. No. IX.—The Two Crappies. BY FRED MATHER. Fishing In Tree Tops. Here a short rod, say 8ft., is long enough, and the line should not be much longer than the rod. A reel is not [...] Read more →
by John Partridge,drawing,1825 From the work of Sir Charles Lock Eastlake entitled Materials for a history of oil painting, (London: Longman, Brown, Green, and Longmans, 1846), we learn the following: The effect of oil at certain temperatures, in penetrating “the minute pores of the amber” (as Hoffman elsewhere writes), is still more [...] Read more →
Buying a book for a serious collector with refined tastes can be a daunting task. However, there is one company that publishes some of the finest reproduction books in the world, books that most collectors wouldn’t mind having in their collection no matter their general preference or specialty. Read more →
William Wyggeston’s chantry house, built around 1511, in Leicester: The building housed two priests, who served at a chantry chapel in the nearby St Mary de Castro church. It was sold as a private dwelling after the dissolution of the chantries. A Privately Built Chapel Chantry, chapel, generally within [...] Read more →
If ever it could be said that there is such a thing as miracle healing soil, Ivan Sanderson said it best in his 1965 book entitled Ivan Sanderson’s Book of Great Jungles. Sanderson grew up with a natural inclination towards adventure and learning. He hailed from Scotland but spent much [...] Read more →
Take to every quart of water one pound of Malaga raisins, rub and cut the raisins small, and put them to the water, and let them stand ten days, stirring once or twice a day. You may boil the water an hour before you put it to the raisins, and let it [...] Read more →
Home Top of Pg. Read more →
This massive volume gives one a real visual sense of what it was like running a highly efficient colonial operation in the early 20rh Century. It will also go a long way to help anyone wishing to understand modern political intrigue in the Middle-East. Click here to read A Survey of Palestine [...] Read more →
WIPO HQ Geneva UNITED STATES PLANT VARIETY PROTECTION ACT TITLE I – PLANT VARIETY PROTECTION OFFICE Chapter Section 1. Organization and Publications . 1 2. Legal Provisions as to the Plant Variety Protection Office . 21 3. Plant Variety Protection Fees . 31 CHAPTER 1.-ORGANIZATION AND PUBLICATIONS Section [...] Read more →
Book Conservators, Mitchell Building, State Library of New South Wales, 29.10.1943, Pix Magazine The following is taken verbatim from a document that appeared several years ago in the Maine State Archives. It seems to have been removed from their website. I happened to have made a physical copy of it at the [...] Read more →
NAPOLEON’S PHARMACISTS. Of the making of books about Napoleon there is no end, and the centenary of his death (May 5) is not likely to pass without adding to the number, but a volume on Napoleon”s pharmacists still awaits treatment by the student in this field of historical research. There [...] Read more →
A Lecture Delivered at the Guildhall, March 2, 1853 by Rev. H.M. Scarth, M.A., Rector of Bathwick. To understand the ancient history of the country in which we live, to know something of the arts and manners of the people who have preceded us, to ascertain what we owe [...] Read more →
Note on Watercolour: F.A. Molony (fl. 1930-1938) was a Major in the Royal Engineers. The National Army Museum hold his work. His work was also shown at an exhibition of officers work at the R.B.A. Galleries (Army Officers’ Art Society) Description from Youtube: June 2015 will see [...] Read more →
Home Top of Pg. Read more →
For years in the West African nation of Ghana medicine men have used a root and leaves from a plant called nibima(Cryptolepis sanguinolenta) to kill the Plasmodium parasite transmitted through a female mosquito’s bite that is the root cause of malaria. A thousand miles away in India, a similar(same) plant [...] Read more →
Click here to access the Internet Archive of old Popular Mechanics Magazines – 1902-2016 Click here to view old Popular Mechanics Magazine Covers Home Top of Pg. Read more →
Jul. 30, 1898 Forest and Stream Pg. 87 Indian Mode of Hunting. I.—Beaver. Wa-sa-Kejic came over to the post early one October, and said his boy had cut his foot, and that he had no one to steer his canoe on a proposed beaver hunt. Now [...] Read more →
A CROCK OF SQUIRREL 4 young squirrels – quartered Salt & Pepper 1 large bunch of fresh coriander 2 large cloves of garlic 2 tbsp. salted sweet cream cow butter ¼ cup of brandy 1 tbsp. turbinado sugar 6 fresh apricots 4 strips of bacon 1 large package of Monterrey [...] Read more →
Banana Propagation Reprinted from the International Institute of Tropical Agriculture (IITA.org) The traditional means of obtaining banana planting material (“seed”) is to acquire suckers from one’s own banana garden, from a neighbor, or from a more distant source. This method served to spread common varieties around the world and to multiply them [...] Read more →
Home Top of Pg. Read more →
PAINTER-WORK, in the building trade. When work is painted one or both of two distinct ends is achieved, namely the preservation and the coloration of the material painted. The compounds used for painting—taking the word as meaning a thin protective or decorative coat—are very numerous, including oil-paint of many kinds, distemper, whitewash, [...] Read more →
Carya Nuts This Handbook is Published by SLMA or the Southeastern Lumber Manufacturer’s Association Click here to read the handbook or click on the link below for a faster download. Hardwood Handbook Home Top of Pg. Read more →
Home Top of Pg. Read more →
Over the years I have observed a decline in manners amongst young men as a general principle and though there is not one particular thing that may be asserted as the causal reason for this, one might speculate… Self-awareness and being aware of one’s surroundings in social [...] Read more →
Today I shall share a bit of market wisdom that will be hard to swallow for some Rolex owners, especially if they bought their Submariner at the top of the magical Covid Watch Bubble that has now collapsed. History often repeats itself, even in the stock market, but when [...] Read more →
Dr. David Starkey, the UK’s premiere historian, speaks to the modern and fleeting notion of “cancel culture”. Starkey’s brilliance is unparalleled and it has become quite obvious to the world’s remaining Western scholars willing to stand on intellectual integrity that a few so-called “Woke Intellectuals” most certainly cannot undermine [...] Read more →
BLACKBERRY WINE 5 gallons of blackberries 5 pound bag of sugar Fill a pair of empty five gallon buckets half way with hot soapy water and a ¼ cup of vinegar. Wash thoroughly and rinse. Fill one bucket with two and one half gallons of blackberries and crush with [...] Read more →
Dutch artist Herman de Vries – Photo taken by son Vince The two videos below of Herman de Vries at work at the Venice Bienalle 2015 are quite inspiring. So inspiring in fact that I moved into a cave for two weeks and wrote Shakespearean tragedy with charcoal. Filled with great joy [...] Read more →
In July of 1968, the National Aeronautics and Space Administration(NASA), published NASA Technical Report TR R-277 titled Chronological Catalog of Recorded Lunar Events. The catalog begins with the first entry dated November 26th, 1540 at ∼05h 00m: Feature: Region of Calippus2 Description: Starlike appearance on dark side Observer: Observers at Worms Reference: [...] Read more →
Home Top of Pg. Read more →
Home Top of Pg. Read more →
Click here to read the Condon Report Home Top of Pg. Read more →
Home Top of Pg. Read more →
Click here to read the Swiss National Bank’s Chronicle of Monetary Events. Home Top of Pg. Read more →
Home Top of Pg. Read more →
Jul. 23, 1898 Forest and Stream, Pg. 65 Horn Measurements. Editor Forest and Stream: “Record head.” How shamefully this term is being abused, especially in the past three years; or since the giant moose from Alaska made his appearance in public and placed all former records (so far as [...] Read more →
A General Process for Making Wine. Gathering the Fruit Picking the Fruit Bruising the Fruit Vatting the Fruit Vinous Fermentation Drawing the Must Pressing the Must Casking the Must Spirituous Fermentation Racking the Wine Bottling and Corking the Wine Drinking the Wine GATHERING THE FRUIT. It is of considerable consequence [...] Read more →
It is a pity that the traditions and literature in praise of fly fishing have unconsciously hampered instead of expanded this graceful, effective sport. Many a sportsman has been anxious to share its joys, but appalled by the rapture of expression in describing its countless thrills and niceties he has been literally [...] Read more →
Home Top of Pg. Read more →
Traditional British Christmas Pudding Recipe by Pen Vogler from the Charles Dickens Museum Ingredients 85 grams all purpose flour pinch of salt 170 grams Beef Suet 140 grams brown sugar tsp. mixed spice, allspice, cinnamon, cloves, &c 170 grams bread crumbs 170 grams raisins 170 grams currants 55 grams cut mixed peel Gram to [...] Read more →
Muscadine Jelly 6 cups muscadine grape juice 6 cups sugar 1 box Kraft Sure Gel or Ball Fruit Jell Home Top of [...] Read more →
Hernando de Soto (c1496-1542) Spanish explorer and his men torturing natives of Florida in his determination to find gold. Hand-coloured engraving. John Judkyn Memorial Collection, Freshford Manor, Bath The print above depicts Spanish explorer Hernando de Soto and his band of conquistadors torturing Florida natives in order to extract information on where [...] Read more →
Man looks at severed hand and foot….for refusing to climb a tree to cut rubber for King Leopold Click here to read The Crime of the Congo by Arthur Conan Doyle Victim of King Leopold of Belgium Click on the link below for faster download. The [...] Read more →
Chipping a Turpentine Tree DISTILLING TURPENTINE One of the Most Important Industries of the State of Georgia Injuring the Magnificent Trees Spirits, Resin, Tar, Pitch, and Crude Turpentine all from the Long Leaved Pine – “Naval Stores” So Called. Dublin, Ga., May 8. – One of the most important industries [...] Read more →
Home Top of Pg. Read more →
Reprint from The Royal Collection Trust website: Kneller was born in Lubeck, studied with Rembrandt in Amsterdam and by 1676 was working in England as a fashionable portrait painter. He painted seven British monarchs (Charles II, James II, William III, Mary II, Anne, George I and George II), though his [...] Read more →
Home Top of Pg. Read more →
Audubon started to develop a special technique for drawing birds in 1806 a Mill Grove, Pennsylvania. He perfected it during the long river trip from Cincinnati to New Orleans and in New Orleans, 1821. Home Top of [...] Read more →
From A History of Fowling, Being an Account of the Many Curios Devices by Which Wild Birds are, or Have Been, Captured in Different Parts of the World by Rev. H.A. MacPherson, M.A. THE RAVEN (Corvus corax) is generally accredited with a large endowment of mother wit. Its warning [...] Read more →
The Apex Building, headquarters of the Federal Trade Commission, on Constitution Avenue and 7th Streets in Washington, D.C.. The building was designed by Edward H. Bennett under the purview of Secretary of the Treasury Andrew W. Mellon, and was completed in 1938 at a cost of $125 million. Photo by Carol M. Highsmith [...] Read more →
Dec. 10, 1898 Forest and Stream Pg. 477-479 Zulu. The little ship shown in the accompanying plans needs no description, as she speaks for herself, a handsome and shipshape craft that a man may own for years without any fear that she will go to pieces [...] Read more →
Mortlake Tapestries at Chatsworth House Click here to read copy of Daemonologie Home Top of Pg. Read more →
Any prudent investor would jump at the chance to receive a guaranteed 6% dividend for life. So how does one get in on this action? The fact of the matter is…YOU can’t…That is unless you are a shareholder of one of the twelve Federal Reserve Banks and the banks under [...] Read more →
Home Top of Pg. Read more →
Salmon and Sturgeon Caviar – Photo by Thor Salmon caviar was originated about 1910 by a fisherman in the Maritime Provinces of Siberia, and the preparation is a modification of the sturgeon caviar method (Cobb 1919). Salomon caviar has found a good market in the U.S.S.R. and other European countries where it [...] Read more →
The low level of work stoppages of recent years also attests to concern about job security. Testimony of Chairman Alan Greenspan The Federal Reserve’s semiannual monetary policy report Before the Committee on Banking, Housing, and Urban Affairs, U.S. Senate February 26, 1997 Iappreciate the opportunity to appear before this Committee [...] Read more →
Country House Essays has returned after a good long summer holiday. More essays soon. Home Top of Pg. Read more →
Notes on the intaglio processes of the most expensive book on birds available for sale in the world today. The Audubon prints in “The Birds of America” were all made from copper plates utilizing four of the so called “intaglio” processes, engraving, etching, aquatint, and drypoint. Intaglio [...] Read more →
? Home Top of Pg. Read more →
Sennen Cove at Dusk – photo by Jim Champion – 2005 Home Top of Pg. Read more →
Home Top of Pg. Read more →
First Pineapple Grown in England Click here to read an excellent article on the history of pineapple growing in the UK. Should one be interested in serious mass scale production, click here for scientific resources. Growing pineapples in the UK. The video below demonstrates how to grow pineapples in Florida. [...] Read more →
The Diamond Empire Home Top of [...] Read more →
Jan Verkolje Antonie van Leeuwenhoek was the first person to describe gout or uric acid crystals 1679. For one suffering gout, the following vitamins, herbs, and extracts may be worth looking into: Vitamin C Folic Acid – Folic Acid is a B vitamin and is also known as B9 – [Known food [...] Read more →
Dec. 24, 1898 Forest and Stream Pg. 513-514 The Standard Navy Boats. Above we find, The accompanying illustrations show further details of the standard navy boats, the lines of which appeared last week. In all of these boats, as stated previously, the quality of speed has been given [...] Read more →
The magician delighted in exposing spiritualists as con men and frauds. By EDMUND WILSON June 24, 1925 Houdini is a short strong stocky man with small feet and a very large head. Seen from the stage, his figure, with its short legs and its pugilist’s proportions, is less impressive than at close [...] Read more →
Mortlake Tapestries at Chatsworth House Click here to learn more about the Mortlake Tapestries of Chatsworth The Mortlake Tapestries were founded by Sir Francis Crane. From the Dictionary of National Biography, 1885-1900, Volume 13 Crane, Francis by William Prideaux Courtney CRANE, Sir FRANCIS (d. [...] Read more →
Country House Christmas Pudding Ingredients 1 cup Christian Bros Brandy ½ cup Myer’s Dark Rum ½ cup Jim Beam Whiskey 1 cup currants 1 cup sultana raisins 1 cup pitted prunes finely chopped 1 med. apple peeled and grated ½ cup chopped dried apricots ½ cup candied orange peel finely chopped 1 ¼ cup [...] Read more →
Quite possibly, the most agonizing decision being made by Baby Boomers across the nation these days is what to do with all that vintage Hi-fi equipment and boxes full of classic rock and roll cassettes and 8-Tracks. I faced this dilemma head-on this past summer as I definitely wanted in [...] Read more →
The first published illustration of Nicotiana tabacum by Pena and De L’Obel, 1570–1571 (shrpium adversana nova: London). Tobacco can be used for medicinal purposes, however, the ongoing American war on smoking has all but obscured this important aspect of ancient plant. Tobacco is considered to be an indigenous plant of [...] Read more →
Click here to visit the New Yorkshire YouTube channel. Home Top of Pg. Read more →
Here, where these low lush meadows lie, We wandered in the summer weather, When earth and air and arching sky, Blazed grandly, goldenly together. And oft, in that same summertime, We sought and roamed these self-same meadows, When evening brought the curfew chime, And peopled field and fold with shadows. I mind me [...] Read more →
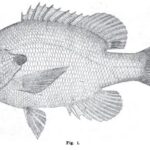
Sept. 3, 1898. Forest and Stream Pg. 188-189 How to Distinguish Fishes. BY FRED MATHER. The average angler knows by sight all the fish which he captures, but ask him to describe one and he is puzzled, and will get off on the color of the fish, which is [...] Read more →
Click here to access the world’s most powerful Import/Export Research Database on the Planet. With this search engine one is able to access U.S. Customs and other government data showing suppliers for any type of company in the United States. Home Top of Pg. Read more →
The following recipes form the most popular items in a nine-course dinner program: BIRD’S NEST SOUP Soak one pound bird’s nest in cold water overnight. Drain the cold water and cook in boiling water. Drain again. Do this twice. Clean the bird’s nest. Be sure [...] Read more →
Photo by Greg O’Beirne Home Top of Pg. Read more →
Home Top of [...] Read more →
Cabildo circa 1936 The Cabildo houses a rare copy of Audubon’s Bird’s of America, a book now valued at $10 million+. Should one desire to visit the Cabildo, click here to gain free entry with a lowcost New Orleans Pass. Home Top of [...] Read more →
Robert W. Service (b.1874, d.1958) There are strange things done in the midnight sun By the men who moil for gold; The Arctic trails have their secret tales That would make your blood run cold; The Northern Lights have seen queer sights, But the queerest they ever did see Was that night [...] Read more →
From Allen’s Indian Mail, December 3rd, 1851 BOMBAY. MUSULMAN FANATICISM. On the evening of November 15th, the little village of Mahim was the scene of a murder, perhaps the most determined which has ever stained the annals of Bombay. Three men were massacred in cold blood, in a house used [...] Read more →
Home Top of Pg. Read more →
Officers and men of the 13th Light Dragoons, British Army, Crimea. Rostrum photograph of photographer’s original print, uncropped and without color correction. Survivors of the Charge. Half a league, half a league, Half a league onward, All in the valley of Death Rode the six hundred. “Forward, the Light Brigade! Charge for the [...] Read more →
Video courtesy of Imperial War Museums, UK Home Top of Pg. Read more →
Home Top of Pg. Read more →
Mudlarks of London Mudlarking along the Thames River foreshore is controlled by the Port of London Authority. According to the Port of London website, two type of permits are issued for those wishing to conduct metal detecting, digging, or searching activities. Standard – allows digging to a depth of 7.5 [...] Read more →
Click here to view Period Furniture Guide Home Top of Pg. Read more →
Home Top of Pg. Read more →
Reprint from The Sportsman’s Cabinet and Town and Country Magazine, Vol I. Dec. 1832, Pg. 94-95 To the Editor of the Cabinet. SIR, Possessing that anxious feeling so common among shooters on the near approach of the 12th of August, I honestly confess I was not able [...] Read more →
Wine Making Grapes are the world’s leading fruit crop and the eighth most important food crop in the world, exceeded only by the principal cereals and starchytubers. Though substantial quantities are used for fresh fruit, raisins, juice and preserves, most of the world’s annual production of about 60 million [...] Read more →
Jujitsu training 1920 in Japanese agricultural school. CHAPTER V THE VALUE OF EVEN TEMPER IN ATHLETICS—SOME OF THE FEATS THAT REQUIRE GOOD NATURE In the writer’s opinion it becomes necessary to make at this point some suggestions relative to a very important part of the training in jiu-jitsu. [...] Read more →
|
PEACH BRANDY 2 gallons + 3 quarts boiled water 3 qts. peaches, extremely ripe 3 lemons, cut into sections 2 sm. pkgs. yeast 10 lbs. sugar 4 lbs. dark raisins Place peaches, lemons and sugar in crock. Dissolve yeast in water (must NOT be to hot). Stir thoroughly. Stir daily for 7 days. Keep [...] Read more →
WITCHCRAFT, SORCERY, MAGIC AND OTHER PSYCHOLOGICAL PHENOMENA AND THEIR IMPLICATIONS ON MILITARY AND PARAMILITARY OPERATIONS IN THE CONGO This report has been prepared in response to a query posed by ODCS/OPS, Department of the Army, regarding the purported use of witchcraft, sorcery, and magic by insurgent elements in the Republic [...] Read more →
Man looks at severed hand and foot….for refusing to climb a tree to cut rubber for King Leopold Click here to read The Crime of the Congo by Arthur Conan Doyle Victim of King Leopold of Belgium Click on the link below for faster download. The [...] Read more →
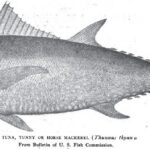
July, 16, l898 Forest and Stream Pg. 48 Tuna and Tarpon. New York, July 1.—Editor Forest and Stream: If any angler still denies the justice of my claim, as made in my article in your issue of July 2, that “the tuna is the grandest game [...] Read more →
The following are transcripts of two letters written by the Founding Father Thomas Jefferson on the subject of seed saving. “November 27, 1818. Monticello. Thomas Jefferson to Henry E. Watkins, transmitting succory seed and outlining the culture of succory.” [Transcript] Thomas Jefferson Correspondence Collection Collection 89 Read more →
Patek Phillipe hand makes the finest watches in the world. Click here to learn more. Home Top of Pg. Read more →
Home Top of Pg. Read more →
The following highly collectible Franklin Library Signed Editions were published between 1977 and 1982. They are all fully leather bound with beautiful covers and contain gorgeous and rich silk moire endpapers. Signatures are protected by unattached tissue inserts. The values listed are average prices that were sought by [...] Read more →
Audubon started to develop a special technique for drawing birds in 1806 a Mill Grove, Pennsylvania. He perfected it during the long river trip from Cincinnati to New Orleans and in New Orleans, 1821. Home Top of [...] Read more →
From Dr. Marvel’s 1929 book entitled Hoodoo for the Common Man, we find his infamous Hoochie Coochie Hex. What follows is a verbatim transcription of the text: The Hoochie Coochie Hex should not be used in conjunction with any other Hexes. This can lead to [...] Read more →
Note on Watercolour: F.A. Molony (fl. 1930-1938) was a Major in the Royal Engineers. The National Army Museum hold his work. His work was also shown at an exhibition of officers work at the R.B.A. Galleries (Army Officers’ Art Society) Description from Youtube: June 2015 will see [...] Read more →
Painting the Brooklyn Bridge, Photo by Eugene de Salignac , 1914 Excerpt from: The Preservation of Iron and Steel Structures by F. Cosby-Jones, The Mechanical Engineer January 30, 1914 Painting. This is the method of protection against corrosion that has the most extensive use, owing to the fact that [...] Read more →
Home Top of Pg. Read more →
THE HATHA YOGA PRADIPIKA Translated into English by PANCHAM SINH Panini Office, Allahabad [1914] INTRODUCTION. There exists at present a good deal of misconception with regard to the practices of the Haṭha Yoga. People easily believe in the stories told by those who themselves [...] Read more →
Photo Caption: The Marquis of Zetland, KC, PC – otherwise known as Lawrence Dundas Son of: John Charles Dundas and: Margaret Matilda Talbot born: Friday 16 August 1844 died: Monday 11 March 1929 at Aske Hall Occupation: M.P. for Richmond Viceroy of Ireland Vice Lord Lieutenant of North Yorkshire Lord – in – Waiting [...] Read more →
IT requires a far search to gather up examples of furniture really representative in this kind, and thus to gain a point of view for a prospect into the more ideal where furniture no longer is bought to look expensively useless in a boudoir, but serves everyday and commonplace need, such as [...] Read more →
Early Texas photo of Tarpon catch – Not necessarily the one mentioned below… July 2, 1898. Forest and Stream Pg.10 Texas Tarpon. Tarpon, Texas.—Mr. W. B. Leach, of Palestine, Texas, caught at Aransas Pass Islet, on June 14, the largest tarpon on record here taken with rod and reel. The [...] Read more →
The greatest cause of failure in vinegar making is carelessness on the part of the operator. Intelligent separation should be made of the process into its various steps from the beginning to end. PRESSING THE JUICE The apples should be clean and ripe. If not clean, undesirable fermentations [...] Read more →
Over the years I have observed a decline in manners amongst young men as a general principle and though there is not one particular thing that may be asserted as the causal reason for this, one might speculate… Self-awareness and being aware of one’s surroundings in social [...] Read more →
by John Partridge,drawing,1825 From the work of Sir Charles Lock Eastlake entitled Materials for a history of oil painting, (London: Longman, Brown, Green, and Longmans, 1846), we learn the following: The effect of oil at certain temperatures, in penetrating “the minute pores of the amber” (as Hoffman elsewhere writes), is still more [...] Read more →
Nov. 12, 1898 Forest and Stream Pg. 396 The Veterans to the Front. Ironton. O., Oct. 28.—Editor Forest and Stream: I mail you a target made here today by Messrs. E. Lawton, G. Rogers and R. S. Dupuy. Mr. Dupuy is seventy-four years old, Mr. Lawton seventy-two. Mr. Rogers [...] Read more →
EBAY’S FRAUD PROBLEM IS GETTING WORSE EBay has had a problem with fraudulent sellers since its inception back in 1995. Some aspects of the platform have improved with algorithms and automation, but others such as customer service and fraud have gotten worse. Small sellers have definitely been hurt by eBay’s [...] Read more →
Sebastian Cox is one of the UK’s premier custom furniture makers with a unique background and love for the forest. Click here to visit SebastianCox.co.uk Home Top of Pg. Read more →
Mudlarks of London Mudlarking along the Thames River foreshore is controlled by the Port of London Authority. According to the Port of London website, two type of permits are issued for those wishing to conduct metal detecting, digging, or searching activities. Standard – allows digging to a depth of 7.5 [...] Read more →
Cleaner for Gilt Frames. Calcium hypochlorite…………..7 oz. Sodium bicarbonate……………7 oz. Sodium chloride………………. 2 oz. Distilled water…………………12 oz. Home Top of Pg. Read more →
” Here’s many a year to you ! Sportsmen who’ve ridden life straight. Here’s all good cheer to you ! Luck to you early and late. Here’s to the best of you ! You with the blood and the nerve. Here’s to the rest of you ! What of a weak moment’s swerve ? [...] Read more →
Twinings London – photo by Elisa.rolle Is the tea in your cup genuine? The fact is, had one been living in the early 19th Century, one might occasionally encounter a counterfeit cup of tea. Food adulterations to include added poisonings and suspect substitutions were a common problem in Europe at [...] Read more →
Jujitsu training 1920 in Japanese agricultural school. CHAPTER V THE VALUE OF EVEN TEMPER IN ATHLETICS—SOME OF THE FEATS THAT REQUIRE GOOD NATURE In the writer’s opinion it becomes necessary to make at this point some suggestions relative to a very important part of the training in jiu-jitsu. [...] Read more →
From The How and When, An Authoritative reference reference guide to the origin, use and classification of the world’s choicest vintages and spirits by Hyman Gale and Gerald F. Marco. The Marco name is of a Chicago family that were involved in all aspects of the liquor business and ran Marco’s Bar [...] Read more →
Take to every quart of water one pound of Malaga raisins, rub and cut the raisins small, and put them to the water, and let them stand ten days, stirring once or twice a day. You may boil the water an hour before you put it to the raisins, and let it [...] Read more →
St.Helen’s on the Thames, photo by Momit From a Dictionary of the Thames from Oxford to the Nore. 1880 by Charles Dickens Abingdon, Berkshire, on the right bank, from London 103 3/4miles, from Oxford 7 3/4 miles. A station on the Great Western Railway, from Paddington 60 miles. The time occupied [...] Read more →
The Racing Knockabout Gosling. Gosling was the winning yacht of 1897 in one of the best racing classes now existing in this country, the Roston knockabout class. The origin of this class dates back about six years, when Carl, a small keel cutter, was built for C. H. [...] Read more →
Hudson Bay: Trappers, 1892. N’Talking Musquash.’ Fur Trappers Of The Hudson’S Bay Company Talking By A Fire. Engraving After A Drawing By Frederic Remington, 1892. Indian Modes of Hunting. IV.—Musquash. In Canada and the United States, the killing of the little animal known under the several names of [...] Read more →
Gold Home Top of Pg. Read more →
The low level of work stoppages of recent years also attests to concern about job security. Testimony of Chairman Alan Greenspan The Federal Reserve’s semiannual monetary policy report Before the Committee on Banking, Housing, and Urban Affairs, U.S. Senate February 26, 1997 Iappreciate the opportunity to appear before this Committee [...] Read more →
Home Top of Pg. Read more →
It is a pity that the traditions and literature in praise of fly fishing have unconsciously hampered instead of expanded this graceful, effective sport. Many a sportsman has been anxious to share its joys, but appalled by the rapture of expression in describing its countless thrills and niceties he has been literally [...] Read more →
Foie gras with Sauternes, Photo by Laurent Espitallier As an Appetizer Pale dry Sherry, with or without bitters, chilled or not. Plain or mixed Vermouth, with or without bitters. A dry cocktail. With Oysters, Clams or Caviar A dry flinty wine such as Chablis, Moselle, Champagne. Home Top of [...] Read more →
Jan Verkolje Antonie van Leeuwenhoek was the first person to describe gout or uric acid crystals 1679. For one suffering gout, the following vitamins, herbs, and extracts may be worth looking into: Vitamin C Folic Acid – Folic Acid is a B vitamin and is also known as B9 – [Known food [...] Read more →
“The Leda, in the Colonna palace, by Correggio, is dead-coloured white and black, with ultramarine in the shadow ; and over that is scumbled, thinly and smooth, a warmer tint,—I believe caput mortuum. The lights are mellow ; the shadows blueish, but mellow. The picture is painted on panel, in [...] Read more →
? Home Top of Pg. Read more →
Click here to access the Internet Archive of old Popular Mechanics Magazines – 1902-2016 Click here to view old Popular Mechanics Magazine Covers Home Top of Pg. Read more →
Paul Thorpe, Brighton, U.K. The YouTube watch collecting world is rather tight-knit and small, but growing, as watches became a highly coveted commodity during the recent world-wide pandemic and fueled an explosion of online watch channels. There is one name many know, The Time Piece Gentleman. This name for me [...] Read more →
Laurens’ portrait as painted during his time spent imprisoned in the Tower of London, where he was kept for over a year after being captured at sea while serving as the United States minister to the Netherlands during the Revolutionary War. The first Christian white man to be cremated in America was [...] Read more →
Are you considering purchasing a copper water pitcher for storing drinking water but have questions about the effects on your health? The following study may help jump-start your research. Storing Drinking-water in Copper pots Kills Contaminating Diarrhoeagenic Bacteria ABSTRACT Microbially-unsafe water is [...] Read more →
VITRUVIUS The Ten Books on Architecture TRANSLATED By MORRIS HICKY MORGAN, PH.D., LL.D. LATE PROFESSOR OF CLASSICAL PHILOLOGY IN HARVARD UNIVERSITY WITH ILLUSTRATIONS AND ORIGINAL DESINGS PREPARED UNDER THE DIRECTION OF HERBERT LANGFORD WARREN, A.M. NELSON ROBINSON JR. PROFESSOR OF ARCHITECTURE IN HARVARD [...] Read more →
THE sense of a consecutive tradition has so completely faded out of English art that it has become difficult to realise the meaning of tradition, or the possibility of its ever again reviving; and this state of things is not improved by the fact that it is due to uncertainty of purpose, [...] Read more →
Gate of Honour, Caius Court, Gonville & Caius Gonville & Caius College, known as Caius and pronounced keys was founded in 1348 by Edmund Gonville, the Rector of Terrington St Clement in Norfolk. The first name was thus Goville Hall and it was dedicated to the Annunciation of the Blessed Virgin Mary. [...] Read more →
Home Top of Pg. Read more →
Home Top of [...] Read more →
Resolution adapted at the New Orleans Convention of the American Institute of Banking, October 9, 1919: “Ours is an educational association organized for the benefit of the banking fraternity of the country and within our membership may be found on an equal basis both employees and employers; [...] Read more →
Hernando de Soto (c1496-1542) Spanish explorer and his men torturing natives of Florida in his determination to find gold. Hand-coloured engraving. John Judkyn Memorial Collection, Freshford Manor, Bath The print above depicts Spanish explorer Hernando de Soto and his band of conquistadors torturing Florida natives in order to extract information on where [...] Read more →
BEEF JERKY Preparation. Slice 5 pounds lean beef (flank steak or similar cut) into strips 1/8 to 1/4 inch thick, 1 to 2 inches wide, and 4 to 12 inches long. Cut with grain of meat; remove the fat. Lay out in a single layer on a smooth clean surface (use [...] Read more →
Home Top of Pg. Read more →
John Atkinson Grimshaw – Glasgow Saturday Night Home Top of Pg. Read more →
Click here to read The First Greek Book by John Williams White The First Greek Book - 15.7MB IN MEMORIAM JOHN WILLIAMS WHITE The death, on May 9, of John Williams White, professor of Greek in Harvard University, touches a large number of classical [...] Read more →
BLACKBERRY WINE 5 gallons of blackberries 5 pound bag of sugar Fill a pair of empty five gallon buckets half way with hot soapy water and a ¼ cup of vinegar. Wash thoroughly and rinse. Fill one bucket with two and one half gallons of blackberries and crush with [...] Read more →
If a Woman asks you to Change, Politely Excuse Yourself and Walk out the Door; Forever Nobody changes; character is built early in life, and by the time one is involved in adult relationships, it is highly unlikely that one can rebuild one’s character. Recognizing this early on in ones adult [...] Read more →
Tom Oates, aka Nabokov at en.wikipedia No two commercial tuna salads are prepared by exactly the same formula, but they do not show the wide variety characteristic of herring salad. The recipe given here is typical. It is offered, however, only as a guide. The same recipe with minor variations to suit [...] Read more →
EIGHTEEN GALLONS is here give as a STANDARD for all the following Recipes, it being the most convenient size cask to Families. See A General Process for Making Wine If, however, only half the quantity of Wine is to be made, it is but to divide the portions of [...] Read more →
German made shotguns by Krieghoff, founded in 1886. Home Top of Pg. Read more →
Silverfish damage to book – photo by Micha L. Rieser The beauty of hunting silverfish is that they are not the most clever of creatures in the insect kingdom. Simply take a small clean glass jar and wrap it in masking tape. The masking tape gives the silverfish something to [...] Read more →
To Clean Watch Chains. Gold or silver watch chains can be cleaned with a very excellent result, no matter whether they may be matt or polished, by laying them for a few seconds in pure aqua ammonia; they are then rinsed in alcohol, and finally. shaken in clean sawdust, free from sand. [...] Read more →
A General Process for Making Wine. Gathering the Fruit Picking the Fruit Bruising the Fruit Vatting the Fruit Vinous Fermentation Drawing the Must Pressing the Must Casking the Must Spirituous Fermentation Racking the Wine Bottling and Corking the Wine Drinking the Wine GATHERING THE FRUIT. It is of considerable consequence [...] Read more →
Felix Weihs de Weldon, age 96, died broke in the year 2003 after successive bankruptcies and accumulating $4 million dollars worth of debt. Most of the debt was related to the high cost of love for a wife living with Alzheimer’s. Health care costs to maintain his first wife, Margot, ran $500 per [...] Read more →
Traditional British Christmas Pudding Recipe by Pen Vogler from the Charles Dickens Museum Ingredients 85 grams all purpose flour pinch of salt 170 grams Beef Suet 140 grams brown sugar tsp. mixed spice, allspice, cinnamon, cloves, &c 170 grams bread crumbs 170 grams raisins 170 grams currants 55 grams cut mixed peel Gram to [...] Read more →
Blackbeard’s Jolly Roger If you’re looking for that most refreshing of summertime beverages for sipping out on the back patio or perhaps as a last drink before walking the plank, let me recommend my Blunderbuss Mai Tai. I picked up the basics to this recipe over thirty years ago when holed up [...] Read more →
Furniture Polishing Cream. Animal oil soap…………………….1 onuce Solution of potassium hydroxide…. .5 ounces Beeswax……………………………1 pound Oil of turpentine…………………..3 pints Water, enough to make……………..5 pints Dissolve the soap in the lye with the aid of heat; add this solution all at once to the warm solution of the wax in the oil. Beat [...] Read more →
Home Top of Pg. Read more →
Buying a book for a serious collector with refined tastes can be a daunting task. However, there is one company that publishes some of the finest reproduction books in the world, books that most collectors wouldn’t mind having in their collection no matter their general preference or specialty. Read more →
Click here to view Period Furniture Guide Home Top of Pg. Read more →
Life insurance certificate issued by the Yorkshire Fire & Life Insurance Company to Samuel Holt, Liverpool, England, 1851. On display at the British Museum in London. Donated by the ifs School of Finance. Photo by Osama Shukir Muhammed Amin FRCP(Glasg) From How to Make Money; and How to Keep it, Or, Capital and Labor [...] Read more →
To Choose Poultry. When fresh, the eyes should be clear and not sunken, the feet limp and pliable, stiff dry feet being a sure indication that the bird has not been recently killed; the flesh should be firm and thick and if the bird is plucked there should be no [...] Read more →
Notes on the intaglio processes of the most expensive book on birds available for sale in the world today. The Audubon prints in “The Birds of America” were all made from copper plates utilizing four of the so called “intaglio” processes, engraving, etching, aquatint, and drypoint. Intaglio [...] Read more →
Home Top of Pg. Read more →
VHF Marifoon Sailor RT144, by S.J. de Waard RADIO INFORMATION FOR BOATERS Effective 01 August, 2013, the U. S. Coast Guard terminated its radio guard of the international voice distress, safety and calling frequency 2182 kHz and the international digital selective calling (DSC) distress and safety frequency 2187.5 kHz. Additionally, [...] Read more →
Jul. 23, 1898 Forest and Stream, Pg. 65 Horn Measurements. Editor Forest and Stream: “Record head.” How shamefully this term is being abused, especially in the past three years; or since the giant moose from Alaska made his appearance in public and placed all former records (so far as [...] Read more →
Officers and men of the 13th Light Dragoons, British Army, Crimea. Rostrum photograph of photographer’s original print, uncropped and without color correction. Survivors of the Charge. Half a league, half a league, Half a league onward, All in the valley of Death Rode the six hundred. “Forward, the Light Brigade! Charge for the [...] Read more →
For years in the West African nation of Ghana medicine men have used a root and leaves from a plant called nibima(Cryptolepis sanguinolenta) to kill the Plasmodium parasite transmitted through a female mosquito’s bite that is the root cause of malaria. A thousand miles away in India, a similar(same) plant [...] Read more →
THE FIRST step in producing a satisfactory crop of tobacco is to use good seed that is true to type. The grower often can save his own seed to advantage, if he wants to. Before topping is done, he should go over the tobacco field carefully to pick [...] Read more →
The following research discussion is from a study funded by the U.S. National Institute of Health entitled: Boschniakia rossica prevents the carbon tetrachloride-induced hepatotoxicity in rat. It may be of interest to heavy drinkers. Home Top of [...] Read more →
Oh Glorious England, verdant fields and wandering canals… In this wonderful series of videos, the CountryHouseGent takes the viewer along as he chugs up and down the many canals crisscrossing England in his classic Narrowboat. There is nothing like a free man charting his own destiny. Read more →
July 9, 1898. Forest and Stream Pg. 25 Some Notes on American Ship-Worms. [Read before the American Fishes Congress at Tampa.] While we wish to preserve and protect most of the products of our waters, these creatures we would gladly obliterate from the realm of living things. For [...] Read more →
Richard Barker KJ Title Pg. Robert Barker was the printer of the first edition of the King James Bible in 1611. He was the printer to King James I and son of Christopher Barker, printer to Queen Victoria I. Home Top of Pg. Read more →
It was a strange assignment. I picked up the telegram from desk and read it a third time. NEW YORK, N.Y., MAY 9, 1949 HAVE BEEN INVESTIGATING FLYING SAUCER MYSTERY. FIRST TIP HINTED GIGANTIC HOAX TO COVER UP OFFICIAL SECRET. BELIEVE IT MAY HAVE BEEN PLANTED TO HIDE [...] Read more →
Reprint from The Sportsman’s Cabinet and Town and Country Magazine, Vol I. Dec. 1832, Pg. 94-95 To the Editor of the Cabinet. SIR, Possessing that anxious feeling so common among shooters on the near approach of the 12th of August, I honestly confess I was not able [...] Read more →
Home Top of Pg. Read more →
Photo by Rebecca Humann Texas Tea Recipe 2 oz Cuervo Gold Tequila Home Top of [...] Read more →
Eadweard Muybridge was a fascinating character. Click here to learn how Eadweard committed “Justifiable Homicide” after shooting his wife’s lover in 1874. Home Top of Pg. Read more →
The Hunt Saboteur is a national disgrace barking out loud, black mask on her face get those dogs off, get them off she did yell until a swift kick from me mare her voice it did quell and sent the Hunt Saboteur scurrying up vale to the full cry of hounds drowning out her [...] Read more →
Sucker The components of any given market place include both physical structures set up to accommodate trading, and participants to include buyers, sellers, brokers, agents, barkers, pushers, auctioneers, agencies, and propaganda outlets, and banking or transaction exchange facilities. Markets are generally set up by sellers as it is in their [...] Read more →
Home Top of Pg. Read more →
Saint Francis of Assisi, founder of the mendicant Order of Friars Minor, as painted by El Greco. Catholic religious order Catholic religious orders are one of two types of religious institutes (‘Religious Institutes’, cf. canons 573–746), the major form of consecrated life in the Roman Catholic Church. They are organizations of laity [...] Read more →
Gilbert Stewart – Sir Joshua Reynolds SIR JOSHUA REYNOLDS‘ WORKING COLOURS, WITH THE ORDER IN WHICH THEY WERE ARRANGED ON HIS PALLETTE. “For painting the flesh, black, blue black, white, lake, carmine, orpiment, yellow ochre, ultramarine, and varnish. “To lay the [...] Read more →
Wojna Kalmarska – 1611 The Kalmar War From The Historian’s History of the World (In 25 Volumes) by Henry Smith William L.L.D. – Vol. XVI.(Scandinavia) Pg. 308-310 The northern part of the Scandinavian peninsula, as already noticed, had been peopled from the remotest times by nomadic tribes called Finns or Cwenas by [...] Read more →
A la Russie, aux ânes et aux autres – by Chagall – 1911 Marc Chagall is one of the most forged artists on the planet. Mark Rothko fakes also abound. According to available news reports, the art market is littered with forgeries of their work. Some are even thought to be [...] Read more →
John Keats Four Seasons fill the measure of the year; There are four seasons in the mind of man: He has his lusty spring, when fancy clear Takes in all beauty with an easy span; He has his Summer, when luxuriously Spring’s honied cud of youthful thoughts he loves To ruminate, and by such [...] Read more →
The downloadable audio clip is of FDR’s Second Fireside Chat recorded on May 7th, 1933. FDR 2nd Fireside Chat - May 7, 1933 - 18.5MB The transcript that follows is my corrected version of the transcript that is found The American Presidency Project website that was created [...] Read more →
? This video by AT Restoration is the best hands on video I have run across on the basics of classic upholstery. Watch a master at work. Simply amazing. Tools: Round needles: https://amzn.to/2S9IhrP Double pointed hand needle: https://amzn.to/3bDmWPp Hand tools: https://amzn.to/2Rytirc Staple gun (for beginner): https://amzn.to/2JZs3x1 Compressor [...] Read more →
The element copper effectively kills viruses and bacteria. Therefore it would reason and I will assert and not only assert but lay claim to the patents for copper mesh stints to be inserted in the arteries of patients presenting with severe cases of Covid-19 with a slow release dosage of [...] Read more →
Full Cover, rear, spine, and front Published by Piranesi Press in collaboration with Country House Essays, this beautiful paperback version of the King James Bible is now available for $79.95 at Barnes and Noble.com This is a limited Edition of 500 copies Worldwide. Click here to view other classic books [...] Read more →
The Chicago portion of the The Great Train Story at the Museum of Science and Industry in Chicago. by Interiority Home Top of Pg. Read more →
Toxicity of Rhododendron From Countrysideinfo.co.UK “Potentially toxic chemicals, particularly ‘free’ phenols, and diterpenes, occur in significant quantities in the tissues of plants of Rhododendron species. Diterpenes, known as grayanotoxins, occur in the leaves, flowers and nectar of Rhododendrons. These differ from species to species. Not all species produce them, although Rhododendron ponticum [...] Read more →
As reported in the The Colac Herald on Friday July 17, 1903 Pg. 8 under Art Appreciation as a reprint from the Westminster Gazette ART APPRECIATION IN THE COMMONS. The appreciation of art as well as of history which is entertained by the average member of the [...] Read more →
The Ardabil Carpet – Made in the town of Ardabil in north-west Iran, the burial place of Shaykh Safi al-Din Ardabili, who died in 1334. The Shaykh was a Sufi leader, ancestor of Shah Ismail, founder of the Safavid dynasty (1501-1722). While the exact origins of the carpet are unclear, it’s believed to have [...] Read more →
Home Top of Pg. Read more →
Cabildo circa 1936 The Cabildo houses a rare copy of Audubon’s Bird’s of America, a book now valued at $10 million+. Should one desire to visit the Cabildo, click here to gain free entry with a lowcost New Orleans Pass. Home Top of [...] Read more →
The following recipes are from a small booklet entitled 500 Delicious Salads that was published for the Culinary Arts Institute in 1940 by Consolidated Book Publishers, Inc. 153 N. Michigan Ave., Chicago, Ill. If you have been looking for a way to lighten up your salads and be free of [...] Read more →
July 2, 1898 Forest and Stream, Fresh-Water Angling. No. IX.—The Two Crappies. BY FRED MATHER. Fishing In Tree Tops. Here a short rod, say 8ft., is long enough, and the line should not be much longer than the rod. A reel is not [...] Read more →
Click here to visit Ovation Guitars Ovation Patent Drawing 1975 Click here to read a copy of the 1975 Patent for the Ovation Guitar Home Top of Pg. Read more →
Royal Exchange and The Bank of England From How to Make Money; and How to Keep it, Or, Capital and Labor based on the works of Thomas A. Davies Revised & Rewritten with Additions by Henry A. Ford A.M. – 1884 CHAPTER XXVI BANKING AND INSURANCE. I [...] Read more →
Home Top of Pg. Read more →
Quite possibly, the most agonizing decision being made by Baby Boomers across the nation these days is what to do with all that vintage Hi-fi equipment and boxes full of classic rock and roll cassettes and 8-Tracks. I faced this dilemma head-on this past summer as I definitely wanted in [...] Read more →
NEWSPAPER.-Printed sheets published at stated intervals, chiefly for the purpose of conveying intelligence on current events. The Romans wrote out an account of the most memorable occurrences of the day, which were sent to public officials. They were entitled Acta Durna, and read substantially like the local column of a [...] Read more →
Edwin Austin Abbey. King Lear, Act I, Scene I (Cordelia’s Farewell) The Metropolitan Museum of Art. Dates: 1897-1898 Dimensions: Height: 137.8 cm (54.25 in.), Width: 323.2 cm (127.24 in.) Medium: Painting – oil on canvas Home Top of Pg. Read more →
From the classic British Movie, The Shooting Party, a 1985 British drama film directed by Alan Bridges based on Isabel Colegate’s 9th novel of the same name published in 1980 we find a scene set in the billiards parlor whereupon the host of the weekend shooting party Sir Randolph Nettleby walks in [...] Read more →
Linseed oil is readily available in many oil painters’ studios. Yardley London Shea Butter Soap can be purchased from a dollar store or pound shop on the cheap. These two ingredients make for the basis of an excellent cleaning system for cleaning oil painting brushes. Home Top of [...] Read more →
The Diamond Empire Home Top of [...] Read more →
Biograph Theater, where John Dillinger was gunned down by the FBI on July 22, 1934 The Great Depression was on—highway based crime was rampant, the gangsters dressed as well as the bankers they robbed, and and Henry Ford’s big beautiful V8 sedan was the getaway car of choice for both wheelman and [...] Read more →
H. M. Scarth, Rector of Wrington By the death of Mr. Scarth on the 5th of April, at Tangier, where he had gone for his health’s sake, the familiar form of an old and much valued Member of the Institute has passed away. Harry Mengden Scarth was bron at Staindrop in Durham, [...] Read more →
Home Top of Pg. Read more →
WIPO HQ Geneva UNITED STATES PLANT VARIETY PROTECTION ACT TITLE I – PLANT VARIETY PROTECTION OFFICE Chapter Section 1. Organization and Publications . 1 2. Legal Provisions as to the Plant Variety Protection Office . 21 3. Plant Variety Protection Fees . 31 CHAPTER 1.-ORGANIZATION AND PUBLICATIONS Section [...] Read more →
Home Top of Pg. Read more →
Reprint from the Sportsman Cabinet and Town & Country Magazine, Vol.1, Number 1, November 1832. MR. Editor, Will you allow me to inquire, through the medium of your pages, the correct meaning of the term thorough-bred fox-hound? I am very well aware, that the expression is in common [...] Read more →
THE SNIPE, from the Shooter’s Guide by B. Thomas – 1811 AFTER having given a particular description of the woodcock, it will only. be necessary to observe, that the plumage and shape of the snipe is much the same ; and indeed its habits and manners sets bear a great [...] Read more →
A Real Soda Jerk FORMULAS FROM VARIOUS SOURCES. Pineapple Frappe. Water, 1 gallon; sugar 2 pounds of water. 61/2 pints, and simple syrup. 2 1/2 pints; 2 pints of pineapple stock or 1 pint of pineapple stock and 1 pint of grated pineapple juice of 6 lemons. Mix, [...] Read more →
Click here to visit Lil’ Lost Lou and purchase a copy of her latest album. Home Top of Pg. Read more →
Roger Scruton – Why Beauty Matters (2009) from Mirza Akdeniz on Vimeo. Click here for another site on which to view this video. Sadly, Sir Roger Scruton passed away a few days ago—January 12th, 2020. Heaven has gained a great philosopher. Home Top of [...] Read more →
Home Top of Pg. Read more →
STORE MANAGEMENT—THE SHIRK. THE shirk is a well-known specimen of the genus homo. His habitat is offices, stores, business establishments of all kinds. His habits are familiar to us, but a few words on the subject will not be amiss. The shirk usually displays activity when the boss is around, [...] Read more →
Kenilworth Abbey Fields – Photo by David Hunt Click here to read Kenilworth by Sir Walter Scott Click here to view Kenilworth Notes Home Top of Pg. Read more →
Here, where these low lush meadows lie, We wandered in the summer weather, When earth and air and arching sky, Blazed grandly, goldenly together. And oft, in that same summertime, We sought and roamed these self-same meadows, When evening brought the curfew chime, And peopled field and fold with shadows. I mind me [...] Read more →
Home Top of Pg. Read more →
Book Conservators, Mitchell Building, State Library of New South Wales, 29.10.1943, Pix Magazine The following is taken verbatim from a document that appeared several years ago in the Maine State Archives. It seems to have been removed from their website. I happened to have made a physical copy of it at the [...] Read more →
It is unnecessary to point out that low-grade fruit may often be used to advantage in the preparation of vinegar. This has always been true in the case of apples and may be true with other fruit, especially grapes. The use of grapes for wine making is an outlet which [...] Read more →
TROF. C. F. HOLDFER AND HIS 183LBS. TUNA, WITH BOATMAN JIM GARDNER. July 2, 1898. Forest and Stream Pg. 11 The Tuna Record. Avalon. Santa Catalina Island. Southern California, June 16.—Editor Forest and Stream: Several years ago the writer in articles on the “Game Fishes of the Pacific Slope,” in [...] Read more →
Banana Propagation Reprinted from the International Institute of Tropical Agriculture (IITA.org) The traditional means of obtaining banana planting material (“seed”) is to acquire suckers from one’s own banana garden, from a neighbor, or from a more distant source. This method served to spread common varieties around the world and to multiply them [...] Read more →
Noel Desenfans and Sir Francis Bourgeois, circa 1805 by Paul Sandby, watercolour on paper The Dulwich Picture Gallery was England’s first purpose-built art gallery and considered by some to be England’s first national gallery. Founded by the bequest of Sir Peter Francis Bourgois, dandy, the gallery was built to display his vast [...] Read more →
A Lecture Delivered at the Guildhall, March 2, 1853 by Rev. H.M. Scarth, M.A., Rector of Bathwick. To understand the ancient history of the country in which we live, to know something of the arts and manners of the people who have preceded us, to ascertain what we owe [...] Read more →
The Queen Elizabeth Trust, or QEST, is an organisation dedicated to the promotion of British craftsmanship through the funding of scholarships and educational endeavours to include apprenticeships, trade schools, and traditional university classwork. The work of QEST is instrumental in keeping alive age old arts and crafts such as masonry, glassblowing, shoemaking, [...] Read more →
Photo by Greg O’Beirne Home Top of Pg. Read more →
To learn more about Julian McDonnell, film director, click here. Home Top of Pg. Read more →
Home Top of Pg. Read more →
Home Top of Pg. Read more →
The arsenicals (compounds which contain the heavy metal element arsenic, As) have a long history of use in man – with both benevolent and malevolent intent. The name ‘arsenic’ is derived from the Greek word ‘arsenikon’ which means ‘potent'”. As early as 2000 BC, arsenic trioxide, obtained from smelting copper, was used [...] Read more →
Video courtesy of Imperial War Museums, UK Home Top of Pg. Read more →
Home Top of Pg. Read more →
Artisans world-wide spend a fortune on commercial brand oil-based gold leaf sizing. The most popular brands include Luco, Dux, and L.A. Gold Leaf. Pricing for quart size containers range from $35 to $55 depending upon retailer pricing. Fast drying sizing sets up in 2-4 hours depending upon environmental conditions, humidity [...] Read more →
Dr. David Starkey, the UK’s premiere historian, speaks to the modern and fleeting notion of “cancel culture”. Starkey’s brilliance is unparalleled and it has become quite obvious to the world’s remaining Western scholars willing to stand on intellectual integrity that a few so-called “Woke Intellectuals” most certainly cannot undermine [...] Read more →
Home Top of Pg. Read more →
|